Frequently Asked Questions
Basics
What is wastewater and environmental surveillance (WES)?
WES involves systematically collecting, processing, and analyzing samples of untreated wastewater, fecal sludge, and fecally-impacted surface waters, followed by the interpretation of the data to support public health. It offers an efficient, cost-effective public health surveillance strategy to track pathogens, pharmaceuticals, illicit drugs, antibiotic resistance genes, and other health markers in a community.
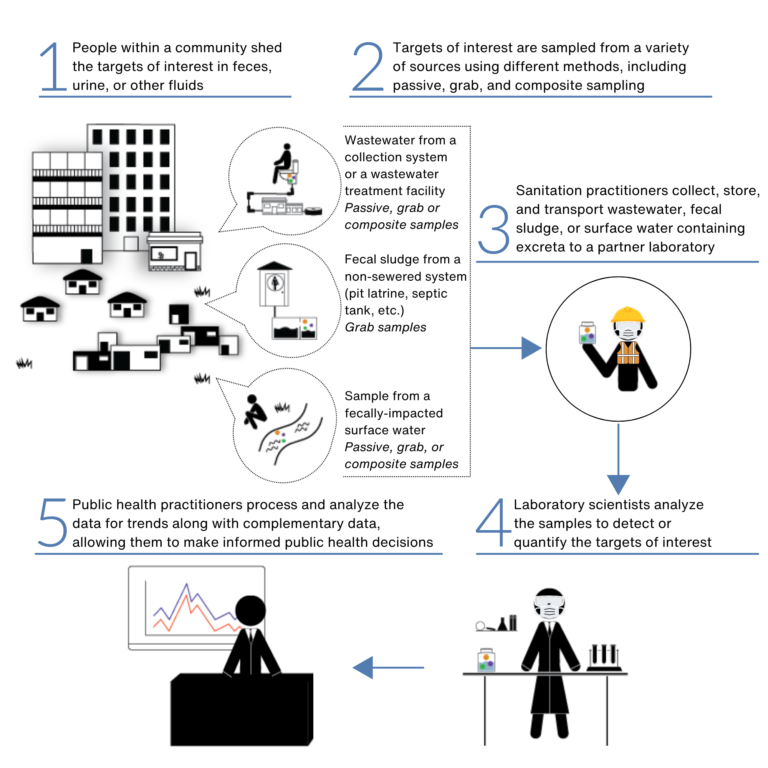
What is the difference between WES, wastewater surveillance, wastewater-based epidemiology (WBE), etc.?
WES, wastewater surveillance, wastewater disease surveillance, and wastewater-based epidemiology (WBE) are all used to describe essentially the same process – the collection, testing, and analysis of samples to understand the presence or trends of certain health-related targets within a community. We have chosen to use WES throughout this website, as it is more inclusive than the other terms, in that it includes the testing of fecal sludge from non-sewered sanitation and environmental samples (i.e. stream and drainageway samples), in addition to wastewater.
Why is the word "surveillance" used?
The use of the term “surveillance” is consistent with the fact that WES is one form of “public health surveillance”, or the “ongoing, systematic collection, analysis, and interpretation of health-related data essential to planning, implementation, and evaluation of public health practice”.
I had never heard of WES before the COVID-19 pandemic. Is it a new concept?
WES is not a new concept, but its use increased dramatically early in the COVID-19 global pandemic when scientists discovered that SARS-CoV-2 could be detected in wastewater (Medema et al. 2020). The origins of WES date back to the first half of the 20th century, with the detection of Salmonella Typhi (Wilson 1928) and poliovirus (Trask & Paul 1942) in wastewater. Since the 1980s, WES has been implemented to systematically detect poliovirus as part of polio eradication efforts (Pöyry et al. 1988; Manor et al. 2014).
What can be detected using WES?
WES can be used to track pathogens (such as Candida auris (Barber et al. 2023; Corrêa et al. 2024), influenza virus (Dlamini et al. 2024; Wolfe et al. 2022), measles virus (Ndlovu et al. 2024), mpox virus (Tiwari et al. 2023; Calabria de Araujo et al. 2024), norovirus (Mabasa et al. 2022), poliovirus (Asghar et al. 2014), respiratory syncytial virus (Hughes et al. 2022), rotavirus (Omatola & Olaniran 2024), Vibrio cholerae (Chigwechokha et al. 2024), and others), pharmaceuticals (Erickson et al. 2021), illicit drugs (Huizer et al. 2021), antibiotic resistance genes (Tiwari et al. 2022), and other health markers that are excreted by the human body. Since WES is a rapidly growing field, scientists are still discovering new applications for WES!
What can the data be used for?
WES data is used by public health professionals alongside other forms of public health surveillance data to inform and evaluate public health actions. These public health actions can include public health communication, vaccination campaigns, mask guidance, coordination with hospitals and other healthcare providers about expected increases in disease transmission, or other measures appropriate for the pathogen or disease of interest. Studies in the U.S. have shown that most adults would consider taking at least one protective health behavior if WES data showed that a virus, such as the flu, was spreading in their area (Soelaeman et al. 2024). WES allows rapid detection and response to emerging infectious disease, making it a useful pandemic prevention tool. Further, as the threat of antimicrobial resistance continues to grow, WES provides a tool for detecting antibiotic resistance genes and their spread.
I want to start a WES program. Where should I start?
Great question! Check out How to Start a WES Program for a step-by-step guide that is relevant for many contexts.
Sampling
Where are samples taken?
WES samples can be collected from:
Untreated wastewater: sewer collection system access points, influent of wastewater treatment plants, and primary settled sludge at wastewater treatment plants
Fecal sludge: septic tanks, pit latrines, and other types of non-sewered sanitation systems
Fecally-impacted surface waters: rivers, streams, and drainageways adjacent to informal settlements or communities without access to safely managed sanitation
What are the different sampling methods?
There are three primary methods for collecting samples for WES – passive samples, grab samples, and composite samples. The descriptions, pros, and cons for each method are shown in the table below:
Sample collection method
Description
Pros
Cons
Passive sample
Adsorbent material, sometimes encased in a plastic “torpedo,” is placed in the wastewater or surface water. It is secured with a string so that it can be retrieved. After a set amount of time, it is removed and brought to the lab so the sample can be processed
- Inexpensive
- Low-theft value
- Minimal maintenance requirements
- Does not require electricity
- Less labor-intensive with no specialized skills required
- Results are difficult to quantify, due to unknown volume flowing through sampler
- The string anchoring the passive sampler can break, and the sampler can be lost
Grab sample
A specific volume of wastewater, surface water, or sludge is collected at a single point in time
- Can be collected quickly using simple equipment
- Inexpensive
- Does not require a power source
- Results are quantifiable
- Highly influenced by changes in flow and composition
- Likely to be less representative than composite samples, particularly when used in smaller systems
Composite sample
Collected by pooling multiple grab samples at a specified frequency over a set time period, often facilitated by an automatic sampler
- Results are quantifiable
- Samples are considered more representative of community fecal contributions than single grab samples
- Sampling is usually automated
- Equipment is expensive and requires a power source (power outlet or battery)
- Equipment can be large and heavy
- Equipment may be susceptible to theft
- Equipment maintenance can be challenging and expensive; parts and service may be hard to acquire in some locations
What personal protective equipment (PPE) should be worn when working with wastewater or fecal sludge?
Those collecting samples should follow occupational safety practices that may include engineering and administrative controls, handwashing, specific safe work practices, and the use of PPE normally required when handling untreated wastewater and fecal sludge.
The Water Environment Federation provides information on safety practices related to specific pathogens, including Candida auris, influenza, and mpox.
How can I transport samples to a laboratory for testing?
If refrigeration is required for the target(s) of interest, samples should be refrigerated as soon as possible during the collection process at temperatures no higher than 4 °C (39 °F) and then transported to the testing location as quickly as possible (same day or next-day) with cold packs. If there is no lab nearby and shipping is required, APHL’s Packing and Shipping Guidance for Biological Substances, Category B Specimens offers instructions on how to package samples for shipment. If possible, samples should be processed within 24 hours of collection to prevent sample degradation and ensure data is actionable.
Analysis
How are samples analyzed?
The required laboratory analysis steps may vary depending on the target of interest, the sample type, and the surveillance program goals.
Analytical methods to test for SARS-CoV-2 in wastewater using polymerase chain reaction (PCR) usually includes inactivation, concentration, extraction, and quantification steps, but these steps may be different for other targets of interest.
If the target of interest needs to be cultured or isolated prior to molecular analysis to be interpreted, then inactivation and concentration steps would likely not occur. Several laboratory methodologies and technologies may exist to process and test samples and can be selected based on cost, supply chain availability, personnel requirements, and other factors.
Visit our WES Techniques page for resources related to sample testing.
What is PCR and how does it work?
Polymerase chain rection (PCR) and reverse transcriptase (RT)-PCR are molecular methods used to detect low levels of nucleic acids specific to public health targets (e.g., SARS-CoV-2 RNA) in wastewater samples. PCR involves amplification of specific gene sequences followed by detection of the amplified gene. PCR methods, including quantitative PCR (qPCR), droplet digital PCR (ddPCR), and digital PCR (dPCR), can be used for both detection and quantification of a specific target.
PCR-based methods have been used for quantification of a wide range of viral pathogens (Blinkova et al. 2009; Hellmer et al. 2014; Boehm et al. 2023), including SARS-CoV-2 (Alygizakis et al. 2021), a diverse range of antibiotic resistance genes (Karkman et al. 2016; Pazda et al. 2019), Candida auris (Barber et al. 2023) in wastewater.
Ethics
What are the primary ethical concerns related to WES?
Ethics related to WES will likely vary by region, but here are some topics to consider when designing a WES program:
- protecting marginalized groups
- protecting community privacy
- considering taboos and cultural norms around handling and testing fecal samples that may influence acceptance of testing
- ensuring WES programs are equitably distributed country-wide
- evaluating who should be included in decision-making and how to involve general community members
- addressing additional considerations that may arise if testing for high-risk substances, including illicit substances
- considering data ownership and who benefits if the data is monetized
- ensuring the purpose of WES is for public health benefit
Are there different ethical considerations for non-sewered vs. sewered settings?
The more people who contribute to the wastewater or fecal sludge at the sample collection point, the more anonymous it will be. While a wastewater sample from a sewered system may represent tens of thousands of individuals in a community, a sample from a non-sewered sanitation system will inherently represent a much smaller number of people. When selecting sampling sites, consider how many people will contribute to that sample. If the number of users is relatively small, extra precautions should be taken to ensure privacy is maintained, particularly in marginalized communities. Consider pooling samples if the contributing population is very small. Sampling from institutional and communal settings may be beneficial for both privacy and sample efficiency purposes.
It is also more likely for a non-sewered system, such as a septic tank or pit latrine, to be located on private property. The WES program should always get permission to take a sample from any system, whether sewered or non-sewered, but this process may be more challenging for non-sewered systems requiring more sample points.
